Why would Mississippi and West Virginia, states that are ranked #1 and #4 in poverty, #5 and #1 in adult obesity, and #2 and #4 in adolescent obesity, respectively, eliminate access to the most affordable version of the safe and effective weight loss drugs Ozempic and Wegovy?
Background on Ozempic and Wegovy
Not since Viagra or Botox, has a class of medications garnered as much attention as Ozempic, Wegovy and Mounjaro. These glucagon-like peptide (GLP-1) receptor agonists mimic naturally occurring gastrointestinal hormones to help suppress appetite and enhance insulin secretion from the pancreas, one of the reasons these medications were first approved for treatment of type 2 diabetes.
During approval studies and real-world treatment with this class of medication, patients with type 2 diabetes were also losing significant amounts of weight. So much so that NovoNordisk, the manufacturer of Ozempic, remarketed the exact same active ingredient, semaglutide, as a chronic weight management medication under the name Wegovy.
Not to be outdone, Eli Lilly, the manufacturer of the type 2 diabetes medication Mounjaro (which mimics both GLP-1 as well the GI hormone gastric inhibitory peptide (GIP)), is in the final stretch of FDA-approval of its active ingredient, tirzepatide, as a weight management medication as well.
The verdict is in. These medications work. Well. Sure there are potential side effects on the gastrointestinal tract such as nausea, vomiting, constipation and diarrhea. But they don’t occur in a majority of patients and they often dissipate with ongoing use. Despite side effects, they’re unquestionably safer than all weight loss drugs that came before. Remember Dexatrim from the ’80’s? Ingredients included the amphetamine-like compound, ephedra. Fen-Phen? Taken off the market in combination due to cardiac issues.
And yes, GLP-1’s have been associated with medullary thyroid cancer in rats but even some of the rats in those early studies that were taking the placebo (inactive medication) as part of the control group developed thyroid cancer. So the association isn’t clear. Additionally, the FDA admits the significance in humans isn’t evident either considering that the incidence of medullary thyroid cancer in humans has been rare and stable for over 30 years while this class of medication has been available for human use for over 20 years. It’s so rare that your risk of dying from medullary thyroid cancer by taking the medication pales in comparison to your risk of dying from heart disease and obesity by not taking the medication.
Medication Shortage
The problem now with such a successful medication on the market is its rampant shortage around the country. Because of that shortage, Ozempic and Wegovy, along with Mounjaro, are on the FDA shortage list. In the case of a shortage, FDA regulations allow compounding pharmacies to make duplicates of these commercially available drugs without fear of patent infringement.
Compounding pharmacies, in contrast to traditional pharmacies, get their materials from FDA-approved manufacturers that follow current Good Manufacturing Practices (cGMP) and customize them for customers. For example, compounding pharmacies can remove a dye from a commercially available drug for someone who is allergic to it. Hospitals have compounding pharmacies that mix specialized medications for patients. And this past winter, compounding pharmacies were encouraged by the FDA to make the liquid form of acetaminophen, the active ingredient in Tylenol, due to a shortage during the recent tripledemic of kids suffering from RSV, flu and COVID in the US.
Similarly, because of the shortage of Ozempic and Wegovy, compounding pharmacies are making a duplicate of the active ingredient, semaglutide. However, semaglutide can come in two forms, the pure base or the salt version (which includes an additional sodium ion attached to the semaglutide amino acid sequence). Because the FDA allows a duplicate of medications on the shortage list, the duplicate must truly be a duplicate of the semaglutide found in Ozempic and Wegovy. NovoNordisk’s original formulation is a semaglutide base, not semaglutide salt. While several compounding pharmacies I spoke with state that the difference between the base and salt form are negligible, the salt form is technically not a duplicate. Regardless, the semaglutide base is being manufactured by FDA-approved manufacturers and is available to compounding pharmacies.
What did the Board of Pharmacy ban in Mississippi and West Virginia?
Rather than the Mississippi and West Virginia Boards of Pharmacy (BOP) ban the use of semaglutide salt, they banned all forms of semaglutide, even the base version that is the active ingredient in the FDA-approved Ozempic and Wegovy.
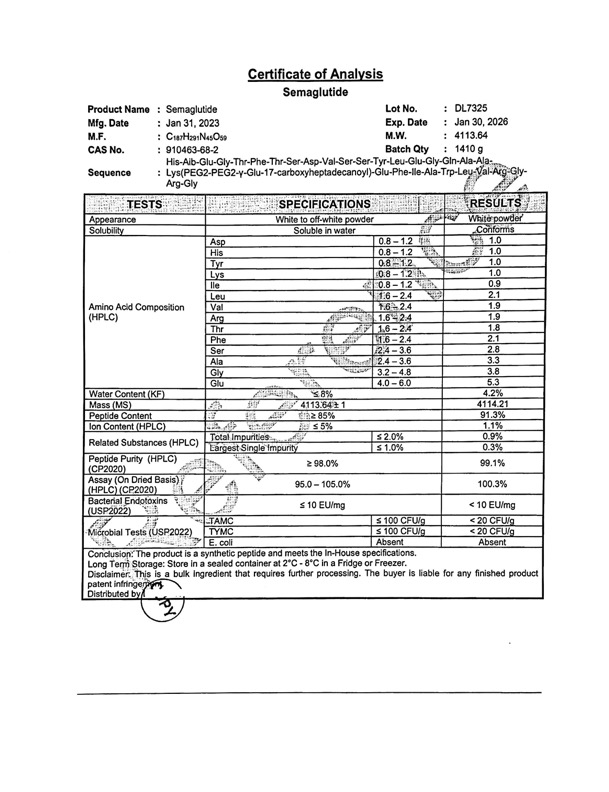
But this suggests these Boards are incapable of differentiating between a compounding pharmacy using the semaglutide base or salt version of the compound. When inspecting and licensing a compounding pharmacy in their state, all raw ingredients are evaluated by the respective BOP and a Certificate of Analysis (CoA) confirms the makeup of the raw ingredients the compounding pharmacy sources from their FDA-approved manufacturer.
For example, the CoA below was provided to me by our compounding pharmacy on behalf of the manufacturer that provided them with the semaglutide base. If this was the salt version, an ‘Na’ would follow the sequence at the top of the document. So if I am able to attain this CoA for my weight management program, you would think a state Board of Pharmacy would be able to do the same and ban only semaglutide salt and not the approved base. If there is something else at play here, no one is owning up to it.
The result: by banning all semaglutide, including the true duplicate of the active ingredient found in Ozempic and Wegovy, a less expensive, more affordable alternative is unavailable for the poor, obese citizens of Mississippi and West Virginia.
But why wouldn’t these Boards perform their due diligence and limit the ban to only semaglutide salt? One plausible answer is NovoNordisk, despite their admission they can’t meet demand, must be behind this campaign. Consider the fact that the letters issued by West Virginia and North Carolina banning all forms of semaglutide are strangely identical. Is NovoNordisk providing templates for pharmacies to ban all versions of the drug leaving a void in the market while they ramp up production? In the absence an alternative explanation, other potentially untrue theories abound.
No comment from NovoNordisk at this time.